Abstract
Adoptive cell therapy with autologous CAR-T cells has induced remarkable responses in patients with treatment-refractory hematologic malignancies, which has led to FDA approvals for two CAR-T products. However, limitations exist with commercial CAR-T centralized production: (1) off-site manufacturing can take several weeks and requires shipping from and to the treating facility; (2) off-site manufacturing limits treatment options for progressing patients; (3) high cost of the commercial products may limit their availability. To address these challenges, we used the fully automated Miltenyi CliniMACS Prodigy device, a GMP-compliant closed system, to manufacture autologous CAR-T cells for a Phase I trial (NCT03019055) evaluating a first-in-human bi-specific CAR that targets CD19 and CD20 (CD20.19 CAR).
CAR-T manufacturing was performed exclusively using the CliniMACS Prodigy device and reagents obtained from Miltenyi Biotec. Production was performed within the Medical College of Wisconsin (MCW) Cell Therapy Laboratory, an ISO7 air handling environment. Manufacturing was set at 14 days, and production was as follows. First, peripheral blood mononuclear cells (MNC) were collected by apheresis, with a collection goal of 4 blood volumes to eliminate risk of a low CD3 yield in heavily pre-treated patients. Next, MNC were loaded onto the Prodigy, and CD4 and CD8 T cells enriched by positive immunomagnetic selection. To start the culture process, enriched T cells were suspended in TexMACS medium supplemented with 3% human AB serum and 200 U/mL IL-2, and TransACT reagent was added to stimulate the T cells in the Prodigy cell culture chamber. The following day (day 1), lentiviral vector expressing anti-CD19 and anti-CD20 (in tandem) with CD3ζ and 4-1BB stimulatory domains was added to the stimulated cells. Culture washes and feedings were done on days 5, 6, 8, 10 and 12 of manufacture, and final products harvested on Day 14. Protein L staining was used to detect expression of CD20.19 CAR on the T cells. On Day 14, eligible patients received fresh CAR-T cells, while for others the product was cryopreserved and administered on a later date.
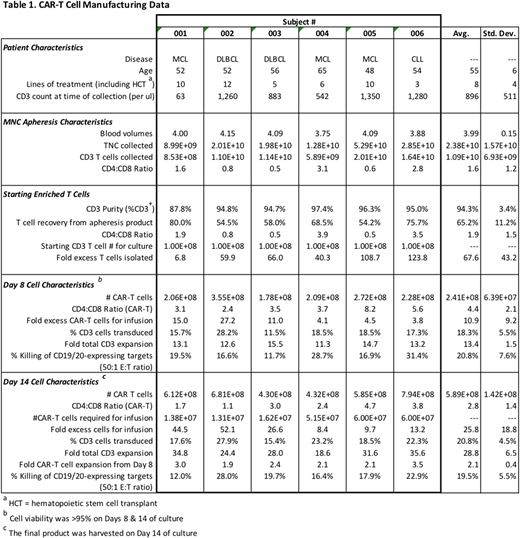
To date, the MCW Cell Therapy Laboratory has successfully generated CAR-T cell products for all 6 patients enrolled thus far on the Phase 1 clinical trial with no production failures (Table 1). Three patients received cryopreserved product and 3 patients received fresh product. The enriched T cells were 94.3% CD3+ (87.8-97.4%), and average T cell recovery from the apheresis cell products was 65.2% (54.2-80.0%). Protein L staining indicated 20.8% average CD20.19 CAR expression. Patient CAR-T cells were able to kill CD19+ and CD20+ target cells in vitro and produce IFN-gamma in response to the same target cells. An average yield of 5.9e+8 (4.3-7.9e+8) CAR T cells was obtained at harvest, which exceeded the required cell dose for all patients. The CAR-T cells were comprised of both CD4 and CD8 T cells, with higher expression on CD4 T cells; average CAR-T CD4:CD8 ratio on the final products was 2.8. The majority of T cells (average of 81.5%) had an effector-memory phenotype. In-process testing performed on Day 8 of manufacturing demonstrated sufficient numbers of CAR-T cells needed for patient infusions were already present, and that the CAR-T cells only expanded an additional 1.9 to 3.5-fold between Days 8 and 14.
In conclusion, we have successfully demonstrated feasibility for point-of-care CAR-T cell production for clinical use from patient apheresis products utilizing the CliniMACS Prodigy device. Time to production was efficient (14 days), and patient-derived CAR-T cell products were reproducibly generated in a standard cell processing laboratory within an academic medical center. A major clinical advantage of CAR-T cells generated on-site is the flexibility in treatment. Patients can receive cells either immediately (i.e., fresh) or the cells can be cryopreserved for later infusion if the patient is not able to receive fresh cells. Based on our results, we intend to decrease the cell processing time to 10 days.
Zhu:Lentigen Technology Inc., A Miltenyi Biotec Company: Research Funding. Shah:Juno Pharmaceuticals: Honoraria; Oncosec: Equity Ownership; Geron: Equity Ownership; Exelexis: Equity Ownership; Miltenyi: Other: Travel funding, Research Funding; Lentigen Technology: Research Funding. Schneider:Lentigen Technology Inc., A Miltenyi Biotec Company: Employment. Keever-Taylor:Medical College of Wisconsin: Research Funding. Dropulic:Lentigen, A Miltenyi Biotec company: Employment. Orentas:Lentigen Technology Inc., A Miltenyi Biotec Company: Employment. Hari:Bristol-Myers Squibb: Consultancy, Research Funding; Amgen Inc.: Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Janssen: Honoraria; Kite Pharma: Consultancy, Honoraria; Takeda: Consultancy, Honoraria, Research Funding; Spectrum: Consultancy, Research Funding; Sanofi: Honoraria, Research Funding. Johnson:Miltenyi: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal